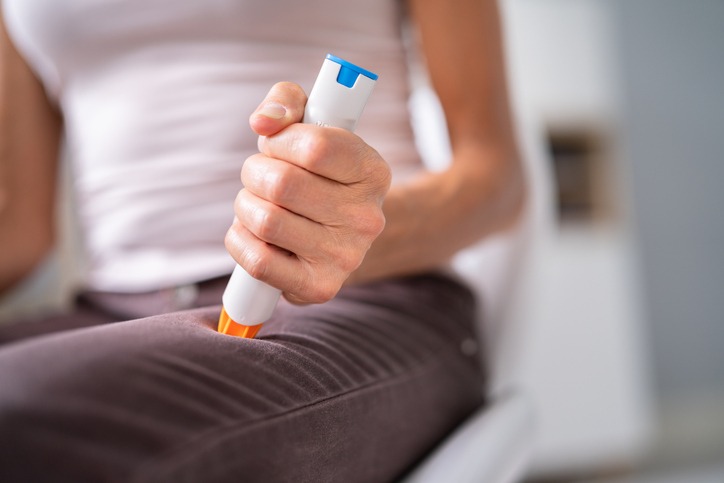
As reported by the BBC, people are being urged to contact their GP and swap brands from the Emerade adrenaline auto-injector pen after faults have been detected
Emerade 300 and 500 microgram auto-injector pens, which treat anaphylaxis, are being recalled after some failed to deliver the dose of adrenaline.
The pens are prescribed for people with severe, life-threatening allergies.
The Medicines and Healthcare products Regulatory Agency (MHRA) says users should ask for alternative brands.
These include EpiPen or Jext. People are also advised to seek help on how to use the replacement pen.
Patients should return their Emerade pens only once they have received a replacement. It is estimated around 25,000 people may have one.
Similar concerns with Emerade pens failing to inject have been raised by the MHRA in the past.
Some of the main triggers of a severe allergic reaction or anaphylaxis requiring adrenaline injections include foods such as nuts and milk, medicines, insect stings and latex.
According to the NHS, adrenaline should be administered if a person starts to feel lightheaded or faint, has breathing difficulties and a fast heartbeat.
Dr Alison Cave, MHRA chief safety officer, said: “We are taking prompt action to protect patients, following detection of damage to internal components of the Emerade pens if they are dropped, which may mean they activate too early or fail to activate and deliver adrenaline.
“Patients are reminded to carry two pens with them at all times as normal and to contact their healthcare professional when a replacement is due.”



Be the first to comment